The global COVID-19 pandemic has returned, and the pandemic alert has once again made all countries suffer. Even China, which has been quiet for a long time, has had small-scale outbreaks in the face of aggressive mutant strains. The new crown vaccine, as an important weapon against the fermentation and strain mutation of the new crown epidemic, has once again become a hot topic of public attention and discussion.
Comparison of the data of 13 approved vaccines in the world, which one is more effective? According to public data, as of May 26, 2021, a total of 442 clinical trials for 185 COVID-19 candidate vaccines have been registered globally, of which 102 (24.5%) trials are in the stage of efficacy confirmation, and 20 (4.8%) The trial is in the post-marketing monitoring stage. According to statistics from Yaozhi data, around the four technological paths of mRNA, adenovirus vector, whole virus inactivation, and recombinant protein, there are currently 13 new coronavirus vaccines approved for marketing or emergency authorization in the world, and there are also a number of research projects. Enter the late stage of clinical trials. All exhibited different clinical protective effects. According to the currently published clinical phase III data, mRNA vaccines have the highest protective effects, reaching about 95%; inactivated vaccines have relatively low protective effects, but they have made great contributions to preventing infection and curbing the spread of the epidemic. contribute. List of new crown vaccines approved for global marketing/emergency use
The inflection point of the epidemic of mutant strains has not yet reached. How protective are the vaccines? However, the new coronavirus is a positive-strand RNA virus, and the genome has a high probability of mutation during replication; in other words, the new coronavirus is prone to mutation. And with the arrival of mutant strains represented by Delta that have high viral load, strong transmission ability, fast transmission speed, and may increase the severity of the disease, "antigen drift" is likely to occur, thereby making the new crown vaccine effective protection rate Decline may not rule out the possibility of failure of existing vaccines; this will undoubtedly become a new challenge and test that needs to be faced in the fight against the epidemic. Therefore, the effectiveness of vaccines in the real world is a hot spot in the study of new coronary pneumonia. The most important thing is the vaccine's ability to prevent infection of the new coronavirus mutant strain. However, because vaccination was mostly started on a large scale at the end of last year, there is not much research in this field, especially under China's good epidemic prevention effect. However, in the real world, research on vaccines for the prevention of new coronavirus mutant strains by companies in various countries in the real world has been ongoing, and it has also demonstrated the good effectiveness of the vaccine. According to a report by Businessinsider, the Pfizer BNT162b2 vaccine (2doses) used in the UK study was 93% effective against the alpha variant and 88% effective against the delta variant; when using the AstraZeneca ChAdOx1nCoV-19 vaccine (2doses), The effectiveness of the Alpha variant is 66%, and the effectiveness of the delta variant is 60%. The effectiveness of various vaccines against new coronavirus variants
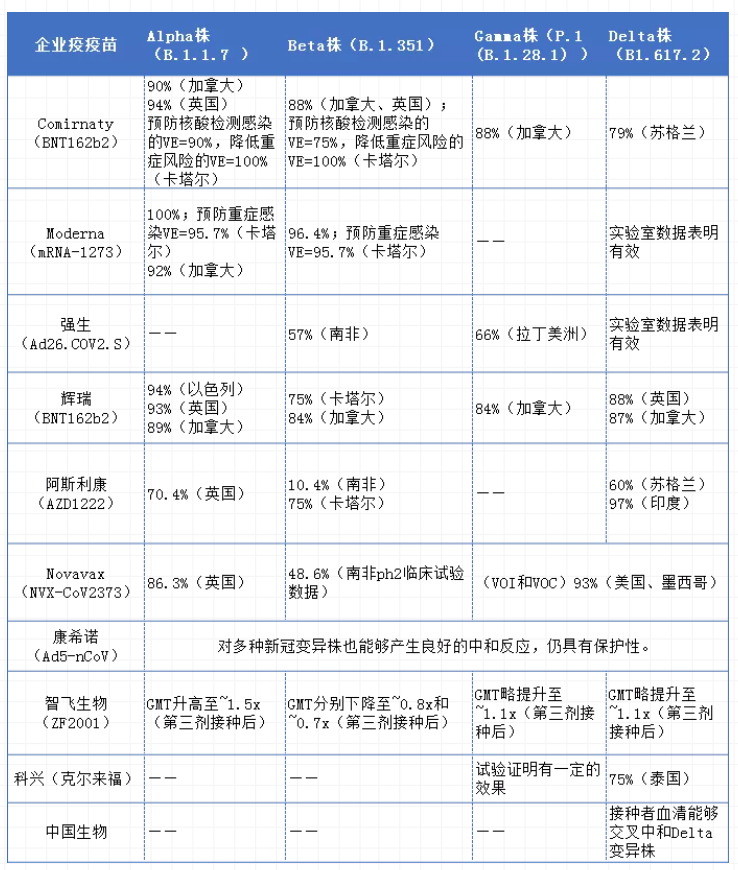
Note: The above data is compiled based on corporate announcements, medRxiv, Businessinsider, Lancet and other announcement materials. If there are omissions, please add them. What about the R&D data? Facing the rapid mutation of the new crown virus, in order to prevent and control the spread of the epidemic, prevent or reduce breakthrough infections, domestic and foreign new crown vaccine manufacturers have promoted the development of vaccines. On August 12, local time in the United States, the US Food and Drug Administration (FDA) announced , To expand the emergency use authorization (EUA) of the mRNA new crown vaccine BNT162b2 jointly developed by Pfizer/BioNTech and the new crown vaccine developed by Moderna, allowing the third dose of booster vaccine to be given to specific immune-compromised people, including patients receiving solid organ transplants And patients who have the same level of immune function due to disease. According to Pfizer's public information, after the third dose of BNT162b2, the neutralizing antibody titer increased by more than 5 times in 18-55-year-old adults, and more than 11-fold in 65-80-year-old adults. . Moreover, the third vaccination has the same tolerance characteristics and good safety as before. Moderna also announced that after the third dose of the booster vaccine, the level of neutralizing antibodies against the new crown variant increased by 30-40 times, and exceeded the level one month after the two doses of vaccine. In fact, companies including Kexing, AstraZeneca, Moderna, and Pfizer have disclosed data on neutralizing antibodies after vaccination. Existing evidence shows that after the completion of the second basic immunization, the third booster shot was vaccinated several months later, the serum antibody level of the vaccinated person increased dozens of times compared with that before the third shot. Comparison chart of booster vaccination data
Reference source: 1. Lancet, doi: 10.1016/S0140-6736(21)00234-8 2. Lancet, doi: 10.1016/S0140-6736(20)32661-1 3. medrxiv, doi: 10.1101/2021.04.27.21256193 4. Public information of research papers on the company's official website, medRxiv, Businessinsider, Lancet, etc.

![]() August 16, 2021
August 16, 2021



