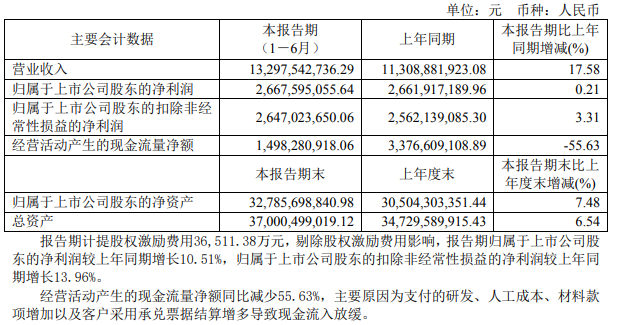
On August 19, Jiangsu Hengrui Pharmaceutical Co., Ltd. (Hengrui Pharmaceutical, 600276) released the first half of 2021 performance report. The financial report shows that in the first half of the year, revenue was 13.298 billion yuan, a year-on-year increase of 17.58%; net profit attributable to shareholders of listed companies was 2.668 billion yuan, a year-on-year increase of 0.21%; net profit attributable to shareholders of listed companies deducting non-recurring gains and losses was 2.647 billion yuan, a year-on-year increase An increase of 3.31%. Regarding the 12.79% revenue growth of the 2020 semi-annual report, Hengrui Pharmaceutical's revenue growth has rebounded in the first half of 2021, but the net profit growth has dropped again. The 2020 mid-year report showed that its net profit attributable to shareholders of listed companies increased by 10.34% year-on-year, and non-net profit increased by 11.94% year-on-year. The 2019 interim report showed that its net profit attributable to shareholders of listed companies increased by 26.32% year-on-year, and non-net profit increased by 25.21% year-on-year.
Innovative drugs contribute 40% of revenue From a specific business perspective, sales of generic drugs have declined, and the proportion of revenue from innovative drugs has increased. During the reporting period, innovative drugs achieved sales revenue of 5.207 billion yuan, a year-on-year increase of 43.80%, accounting for 39.15% of overall sales revenue. The report shows that since 2018, the company has entered the country to purchase a total of 28 varieties of generic drugs, and 18 varieties have been selected. The average price of the selected products has dropped by 72.6%, which has put greater pressure on the company's performance. The 6 drugs involved in the third batch of centralized procurement started in November 2020, the sales revenue fell 57% month-on-month during the reporting period. Regarding the competition of innovative drugs, Hengrui Pharmaceuticals mentioned that the domestic innovative biopharmaceutical industry has serious homogeneity competition, and the costs of R&D, manpower, and production are rising rapidly, and biomedical innovation is facing severe challenges. It is worth noting that the PD-1 anti-tumor drug carrelizumab is the main product of Hengrui Medicine. As of June this year, 6 indications have been approved. The latest interim report did not disclose specific sales data, but mentioned that the negotiated price of medical insurance will be implemented from March 1, 2021, with a reduction of 85%, plus the difficulty of entering the hospital and the different implementation time of medical insurance in various regions. The problem caused a negative growth in the sales revenue of Karelizumab. Hengrui Medicine stated that the company urgently needs to concentrate resources to achieve rapid breakthroughs in innovation and internationalization. R&D investment hits a new high, and the R&D pipeline is open! The report shows that during the reporting period, Hengrui Pharmaceuticals invested 2.581 billion yuan in R&D, an increase of 38.48% year-on-year, and R&D investment accounted for 19.41% of sales revenue, a record high. In the first half of the year, the company obtained a total of 5 production approvals for innovative drug preparations, 9 production approvals for generic pharmaceutical preparations, 41 clinical approvals for drugs and consistency evaluation approvals for 10 varieties, and completed the consistency evaluation application for two products. There are more than 240 clinical projects carried out at home and abroad. At the same time, 131 new domestic patent applications and 39 new international PCT applications were submitted, 64 domestic authorizations and 59 foreign authorizations were obtained. As of the end of the reporting period, the company's R&D pipeline is as follows. (1) Follow-up main clinical R&D pipelines of marketed innovative drugs
(2) Main clinical research and development pipelines of innovative drugs under research
(3) Main clinical research and development pipelines of overseas innovative drugs
23 international clinical trials have been carried out simultaneously, and the overseas market of innovative drugs has gone further In terms of internationalization, Hengrui Pharmaceuticals spent 643 million yuan in overseas R&D in the first half of the year. The report shows that Hengrui has established an overseas R&D team of 136 people in the United States, Europe and other places. During the reporting period, a total of 23 international clinical trials were promoted, including 7 international multi-center phase III projects, and more than 10 research projects were in progress. In the preparation phase, a total of 86 overseas centers were launched. It is worth mentioning that Hengrui Medicine obtained 3 US FDA clinical approvals in the first half of the year. Among them, the international multi-center phase III study of carrelizumab combined with apatinib for the treatment of advanced liver cancer has completed overseas enrollment and launched Preparation work before submission of US FDA BLA/NDA. Based on this research, Carrelizumab has obtained the FDA orphan drug qualification for the treatment of advanced hepatocellular carcinoma indications. It is expected to enjoy certain policy support in the follow-up research and development and commercialization, which will make its overseas listing goal one step closer.

![]() August 24, 2021
August 24, 2021










