Antibody drugs only have a history of more than 30 years, and have entered the outbreak stage in the past five years. By the end of 2020, the US FDA has approved 99 Kinds of antibody drugs on the market. Corresponding to the continuous growth of the number of approved antibody drugs, the global antibody drug market is climbing. The market size will reach US $142.7 billion in 2019 and exceedUS $150 billion in 2020. With the rapid development of double resistance and ADC, there is still a broader market space to be explored.
1、 Antibody drugs approved by FDA in 2020
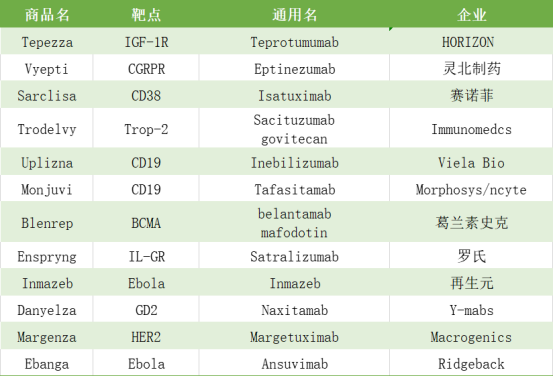
In 2020, the US FDA approved 12 kinds of antibody drugs, among which the IGF-1R antibody tepezza, bcma-adc drug blenrep, trop-2-adc drug trodelvy and Ebola antibody inmazeb are all first in class antibody drugs. At the same time, the number of engineered antibodies increased significantly. Two CD19 antibodies and one HER2 antibody were all engineered antibody drugs based on the old targets.
Table 1: anti body drugs approved by FDA in 2020
2、 Antibody drugs on the market in China in 2020
In 2020, China Drug Administration approved 25 antibody drugs to be listed, including 7 domestic varieties and 18 imported varieties. Among them, vitbutuximab for injection of Wutian
Pharmaceutical is ADC drug, and beilintoumo for injection of amjin is double antibody drug.
Table 2: antibody drugs on the market in China in 2020
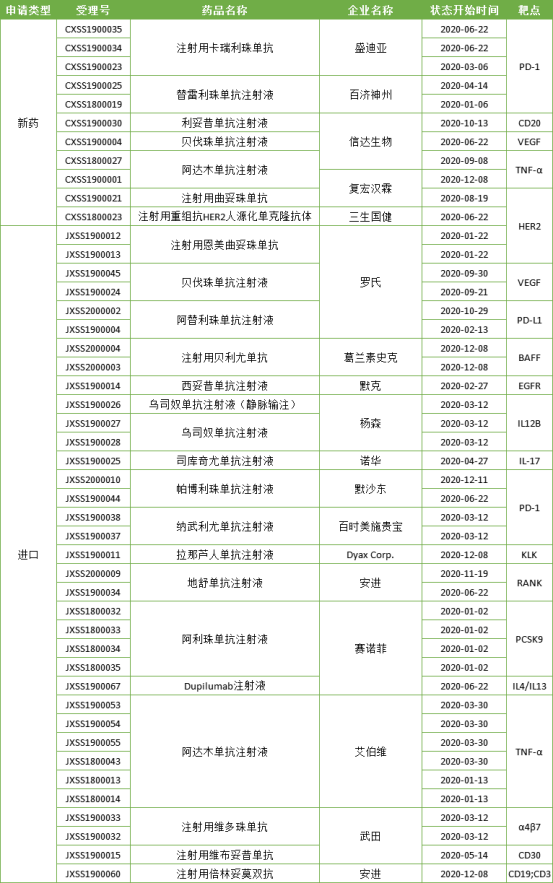
It can be seen from the above table that the approved domestic antibody drugs are still mainly biologically similar drugs, and FIC new drugs are scarce. At the same time, the imported antibody drugs have been approved to the market in China, and the indications have been continuously expanded. The competition between domestic drugs and imported drugs has intensified.
3、 Rapid development of antibody drugs in China
According to the data of drug intelligence, in 2020, CDE undertook 237 clinical applications for antibody drugs, including 208 applications for clinical trials of antibody drugs (173 for monoclonal antibodies, 27 for double antibodies and 8 for ADC drugs), and 29 applications for marketing of antibody drugs.
The hot target track is crowded, and the differentiated innovation is gradually emerging
In 2020, CDE will accept 29 applications for the listing of domestic antibody drugs, including 14 varieties, of which PD-1 / PD-L1 is the most declared target, and weidituximab for Rongchang biological injection is the first domestic ADC drug to be declared for listing.
Table 3: application for marketing of antibody drugs accepted by CDE in 2020

In 2020, CDE will accept 173 applications for clinical trials of domestic monoclonal antibodies based on the acceptance number. The hot targets include PD-1 / PD-L1 (19 varieties), CD47 (10 varieties), IL-17, CTLA4, HER2, claudin 18.2, etc.
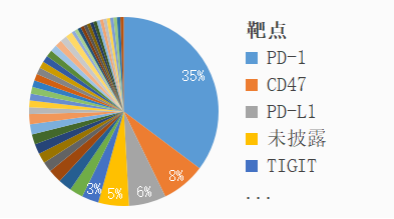
Figure 1: target distribution of clinical monoclonal antibody drugs declared in 2020 (by acceptance number)
Although rapid follow-up is still the main theme of China's innovation, the differentiated innovation achievements of domestic enterprises have gradually emerged. For example, tjc4, a CD47 monoclonal antibody of Tianjing biology, has a unique antigen binding epitope, which can minimize the binding with normal red blood cells and reduce the impact on red blood cells, and has the potential of the best of its kind; in classical Hodgkin's lymphoma, the tirelizumab of Baiji Shenzhou has the highest complete remission rate (CR) among all PD-1 marketed in China. Kangning Jierui's subcutaneous injection of PD-L1 monoclonal antibody kn035 is the first subcutaneous injection of PD-1 / L1 monoclonal antibody submitted to NDA in China.
In addition, there are also innovative pharmaceutical companies committed to developing new target drugs. For example, Dr. Shen Yuelei, founder / Chairman of baccetur, said that he hopes to find new targets and new monoclonal antibodies through the "thousands of mice and ten thousand antibodies" plan.
Dual antibody and ADC drugs become new competitive fields
Although monoclonal antibody is the most popular immunotherapy at present, its curative effect is still unsatisfactory. It is not easy for a monoclonal antibody to achieve the ideal therapeutic effect. In order to achieve a breakthrough, double antibody is a main development direction. In addition, in recent years, ADC technology is gradually mature, or will be another research and development hotspot.
Although the dual antibody and ADC drugs started late in China, good results have been achieved in the number of clinical studies. In 2020, CDE will undertake 27 double antibody clinical applications and 8 ADC clinical applications.
Table 4: clinical applications for dual antibody and ADC drugs accepted by CDE in 2020
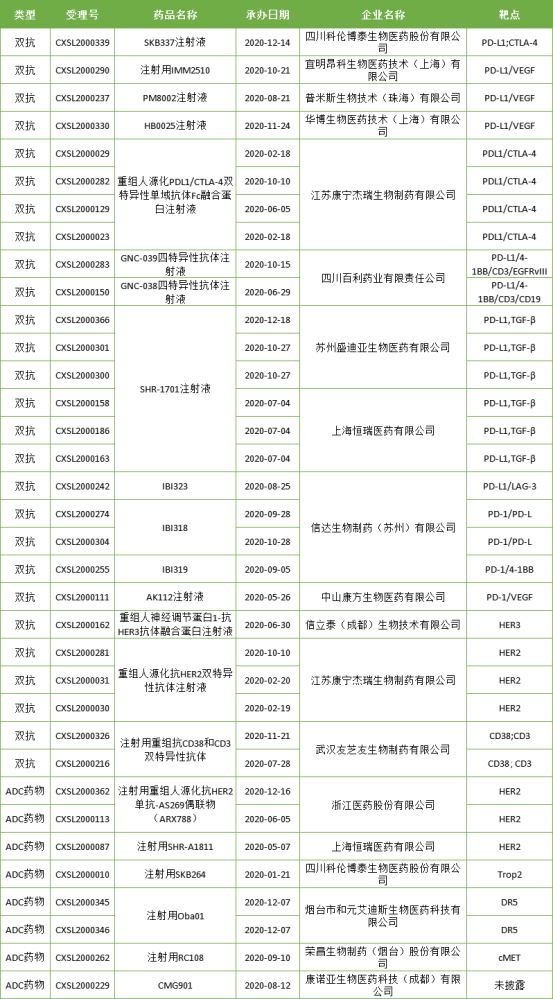
In the field of dual antibody and ADC drugs, the world is in the late clinical stage, and few drugs have been listed. Domestic development enterprises mainly cooperate with multinational companies and Chinese local enterprises. Although most of the domestic dual antibody drugs and ADC drugs are still in the early stage of development, the number of dual antibody drugs developed in China has far exceeded that of multinational enterprises. For example, kn046 of Corning Gerry is the world's first dual antibody against PD-L1 and CTLA-4 at the same time. At present, the phase III clinical trial on the efficacy and safety of kn046 combined with platinum based chemotherapy for stage IV squamous non-small cell lung cancer has been launched in China.
4、 Summary
In recent years, the state has issued a series of policies to regulate imitation, encourage innovation, and speed up the evaluation and approval of innovative drugs through conditional approval for listing, breakthrough therapy, and priority evaluation policies. For antibody drugs, the domestic multi-target new drugs are close to the global R & D speed, and there are a number of global original new drugs.
Dr. Tao Weikang, CEO of Hengrui pharmaceutical R & D center, once said, "China's market has become the second largest pharmaceutical market, but the proportion of innovative drugs in China's pharmaceutical market is extremely low, 76% in developed countries, 27% in emerging countries, and China predicts that it will be less than 25% by 2023. Therefore, in this regard, it should be said that there is still a lot of work and great opportunity for pharmaceutical enterprises Opportunities, opportunities. "
Although the domestic hot target track is crowded, the differentiated innovation is gradually emerging. It is believed that with the continuous increase of R & D projects, China's innovative drugs will make great progress.

![]() January 29, 2021
January 29, 2021





